Ozempic no replacement for willpower when it comes to weight loss

A new meta-study — a study of studies — reveals an inconvenient truth about weight loss itself: Willpower still matters. Manufacturers of GLP-1 injectables like Wegovy and Ozempic would prefer we forget that, since forgetting it is profitable.
The counter-claim — that diets and exercise are no match for our genes and environment — is one fat-positivity influencers have pushed for years. Now it has been eagerly adopted by companies like Novo Nordisk and Eli Lilly to market their new, lizard-venom-derived blockbuster drugs.
People who stop taking weight-loss drugs regain weight at an average rate of 0.4 kilograms per month — roughly 10 pounds per year.
Business is booming. One in eight American adults have taken a weight-loss drug at one time — and this is only the beginning. Uptake remains far below its theoretical ceiling: More than 70% of U.S. adults are overweight or obese, including roughly 40% who are clinically obese.
Shred-pilled?
What comes next is obvious. Adoption will surge as delivery methods improve, especially pills. People don’t like needles. Pills are much easier to swallow.
Just before Christmas, the Food and Drug Administration approved a pill version of Wegovy, imaginatively branded the Wegovy Pill. Pill versions of competing drugs, including Mounjaro, are expected to follow this year.
Some time ago, I predicted that a weight-loss drugmaker would become the largest company in the world within a decade. I made that prediction when Novo Nordisk — the Danish maker of Wegovy and Ozempic — became Europe’s most valuable company, with a market capitalization of roughly $570 billion, more than $200 billion greater than Denmark’s entire GDP. (It has since fallen a few spots.) I now refine that forecast: The pharmaceutical company that perfects the weight-loss pill — balancing results, side effects, and cost — will be the largest company on Earth.
There are already more than one billion obese people worldwide. There is no obvious reason why every one of them couldn’t be prescribed a daily pill.
RELATED: Fat chance! Obese immigrants make America sicker

Chubby checkers
Which brings us back to the meta-study. One of the central unanswered questions surrounding these drugs is what happens when patients stop taking them. Does the weight stay off — or does it return?
In practice, many people don’t stay on them long. Roughly half of users discontinue weight-loss drugs within a year, most often citing cost and side effects, which can include severe gastrointestinal distress, vision problems, and — in rare cases — death.
What happens after discontinuation matters enormously. If the weight returns, many users will be forced to remain on these drugs indefinitely — possibly for decades — to avoid relapse. Pharmaceutical executives have generally been reluctant to acknowledge this implication, though some have done so candidly.
Habit-forming
The researchers behind the new meta-study asked a sharper question still: How does stopping weight-loss drugs compare with stopping traditional interventions like diet and exercise?
The answer is stark. People who stop taking weight-loss drugs regain weight at an average rate of 0.4 kilograms per month — roughly 10 pounds per year. That is four times faster than the weight regain seen in people who stop exercising and restricting calories.
Four times.
The explanation is not mysterious. Pills do not build habits. Diet and exercise do. With drugs, appetite suppression is outsourced to chemistry rather than cultivated through discipline. Remove the compound, and users are left with the same reserves of willpower they had before. Evidence so far suggests that changes to brain chemistry, hormone signaling, and metabolism fade along with the drug itself.
Even when people who diet and exercise relapse, the habits they developed tend to soften the fall. That counts for something.
None of this is to deny that weight-loss drugs can be a valuable tool. For many severely obese people, they may represent the only realistic chance of meaningful weight reduction. If we want to reduce the burden of chronic disease, drugs like Wegovy will have a role to play.
But their rise should not excuse the abandonment of harder truths. Sustainable weight loss still depends on choices, habits, and character — and on reshaping a food environment that makes bad choices effortless and good ones rare. Pharmaceuticals may assist that work. They cannot replace it.
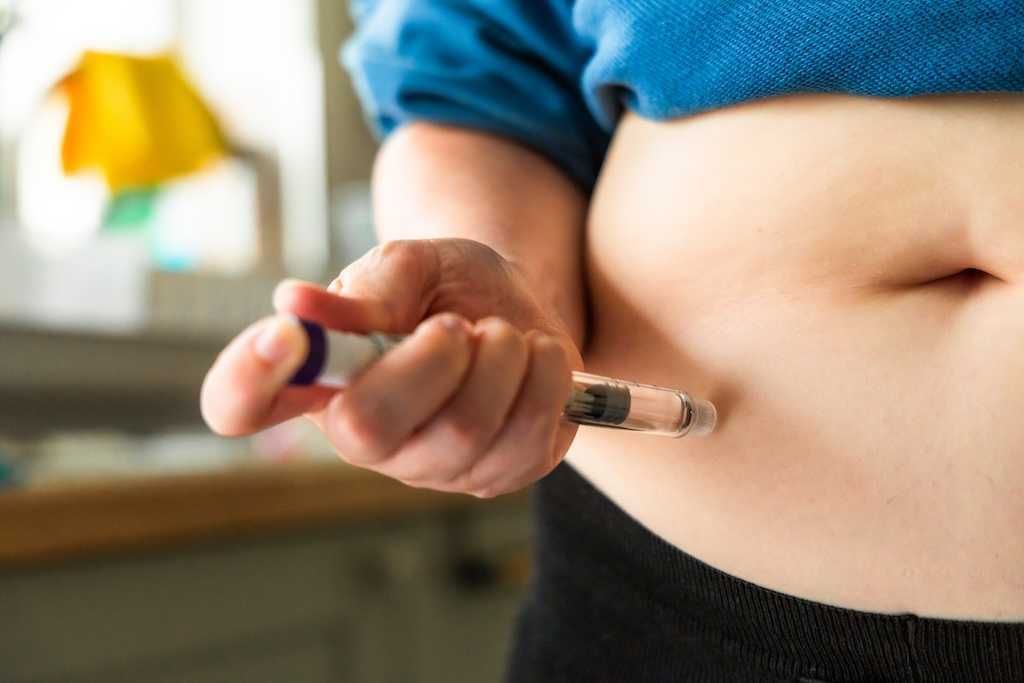
 byemo via iStock/Getty Images
byemo via iStock/Getty Images
 Photo by Brendan SMIALOWSKI / AFP via Getty Images
Photo by Brendan SMIALOWSKI / AFP via Getty Images

 Photo by Andrew Harnik/Getty Images
Photo by Andrew Harnik/Getty Images
 Credit: Photo by Pete Marovich/Getty Images
Credit: Photo by Pete Marovich/Getty Images
 Photo by SAUL LOEB/AFP via Getty Images
Photo by SAUL LOEB/AFP via Getty Images
 Deagreez via iStock/Getty Images
Deagreez via iStock/Getty Images
 Blaze Media
Blaze Media
 Jennifer Kosig via iStock/Getty Images
Jennifer Kosig via iStock/Getty Images