Here Are The 3 Biggest Findings In Florida’s Grand Jury Report On Covid ‘Wrongdoing’
 A new grand jury report contains damning information about how the 'expert' class did not, in fact, 'follow the science' on Covid.
A new grand jury report contains damning information about how the 'expert' class did not, in fact, 'follow the science' on Covid.A new peer-reviewed study found that regular use of ivermectin reduced the risk of dying from COVID-19 by 92%.
The large study was conducted by Flávio A. Cadegiani, MD, MSc, PhD. Cadegiani is a board-certified endocrinologist with a master's degree and doctorate degree in clinical endocrinology.
The peer-reviewed study was published on Wednesday by the online medical journal Cureus. The study was conducted on a strictly controlled population of 88,012 people from the city of Itajaí in Brazil.
Individuals who used ivermectin as prophylaxis or took the medication before being infected by COVID experienced significant reductions in death and hospitalization.
According to the study, those who took ivermectin regularly had a 92% reduction in their COVID death risk compared to non-users and 84% less than irregular users.
"The hospitalization rate was reduced by 100% in regular users compared to both irregular users and non-users," the study stated.
The impressive reduction for regular ivermectin users was evident despite the regular users being at a higher risk for COVID deaths. The regular users were older and had a higher prevalence of type 2 diabetes and hypertension than irregular and non-users.
Irregular users of ivermectin had a 37% lower mortality rate reduction than non-users.
The study defined regular users as those who used more than 30 tablets of ivermectin over five months. The dosage of ivermectin was determined by body weight, but "most of the population used between two and three tablets daily for two days, every 15 days."
"Non-use of ivermectin was associated with a 12.5-fold increase in mortality rate and a seven-fold increased risk of dying from COVID-19 compared to the regular use of ivermectin," the study read. "This dose-response efficacy reinforces the prophylactic effects of ivermectin against COVID-19."
Cadegiani believes the study showed a "dose-response effect" – which means that increasing levels of ivermectin decreased the risk of hospitalization and death from COVID-19.
Cadegiani wrote on Twitter, "An observational study with the size and level of analysis as ours is hardly achieved and infeasible to be conducted as a randomised clinical trial. Conclusions are hard to be refuted. Data is data, regardless of your beliefs."
The war on all information that might help COVID patients survive wasn’t just limited to prescription drugs. There has been a complete blackout on over-the-counter supplements and common medicines that the public is very familiar with and that are readily available, which together could have reduced the mortality burden. A new study published in the heavy-hitting Journal of the American Medical Association demonstrates that it is criminal how to this day our government has failed to advise people on the simple use of aspirin at first sign of COVID.
Like many other people, Advil is my first go-to medication when I feel feverish. Yet when I thought I had COVID in July 2020 (I ultimately didn’t), even this non-doctor was familiar with the already robust academic literature behind the use of aspirin for COVID. So, I went out to the pharmacy and bought some aspirin. Now, a new study published in JAMA by George Washington University researchers shows a modest decrease in mortality just from this well-known over-the-counter drug even in those already hospitalized. Imagine if everyone had been instructed to have it on hand at the first sign of trouble.
The very large retrospective cohort study shows a 13.5% decrease in mortality among hospitalized patients who were administered a baby aspirin per day. The benefit observed was even higher among those with comorbidities. Additionally, and not surprisingly, the study found a 29% decrease in incidence of pulmonary embolism, which is a very common factor landing COVID patients in the ICU. The analysis looked at 112,269 hospitalized patients across 64 different U.S. health systems from January through September 2021. No extra incidence of adverse events was observed in the treatment group.
A separate smaller study conducted by the same lead author published last April of patients in 2020 found an even greater benefit, possibly because the original strain was less aggressive. In the underpowered study, aspirin use was associated with a decreased risk of mechanical ventilation by 44 percent, ICU admission by 43 percent, and in-hospital mortality by 47 percent.
It is criminal that it has taken two years to get out this information. Moreover, these significant but relatively modest results were among those already hospitalized, and the dosage was just 80mg. Most of the doctors who aggressively treated COVID recommend taking aspirin from day one outpatient at the first sign of trouble. One could likely assume that the outcomes would be even better with the higher dose and earlier use before the micro-clotting set in with many hospitalized patients. Dr. Peter McCullough, a well-published cardiologist and internist who developed one of the earliest treatment protocols, noted that daily aspirin was part of his protocol for COVID patients already in 2020.
Chow et al, ANCHOR Investigators, ASA modest late impact. In "McCullough Protocol" since 2020 but much better to start in first three days of illness before microthrombi induced hypoxemia develops. Our dose has been 325 mg given very high UTXA2/B2 found in preclinical studies.pic.twitter.com/cUwSxhl8Gw— Peter McCullough, MD MPH (@Peter McCullough, MD MPH) 1648217958
McCullough also told me that “the fact that aspirin did anything with inpatient mortality given that 98% of those in the study already received Lovenox, a strong blood thinner, speaks to the possibility of early aspirin at home having a much greater chance at beneficial outcomes.”
It should not have taken nearly two years for this information to be published in a high-impact journal, and even then, there are no signs of anyone in the medical establishment or NIH recommending its use.
Although acute COVID seemed to cause severe pulmonary inflammation and fibrosis, it was observed early on in the pandemic that many people ultimately died from blood clotting. This is why Dr. Pierre Kory, co-founder of the FLCCC, tells me, “Aspirin was a part of our early treatment protocol from day one.”
Dr. Bryan Tyson, who treated thousands at his urgent care in El Centro, California, used aspirin at the first stages of the pandemic and advised patients to take it for a while. “We started using full aspirin outpatient on our high-risk patients for 30-60 days depending on risk factors, and we never had a patient develop a pulmonary embolism or deep vein thrombosis or heart attacks,” said one of America’s most prolific COVID doctors. “We knew the spike protein was thrombotic, so we were more apt to use the full dose of aspirin.” Tyson, who is running for Congress in CA-25, believes it is criminal negligence for the medical professional not to pre-emptively treat the thrombosis, which can loom early in the disease and persist for quite some time afterwards.
This demonstrates that the war on hydroxychloroquine and ivermectin had nothing to do with something inherent in those drugs. We have witnessed the same blackout on non-prescription treatments such as NAC, vitamin D, vitamin C, zinc, quercetin, Pepcid, and betadine nasal/oral rinse. Imagine a multipronged approach, with several of these plus some anti-inflammatory prescriptions early on. Well, that’s why Dr. Tyson had such an amazing success rate treating 7,000 patients.
Most of these treatments were downright attacked. Right as some of the studies were coming out about aspirin, the entire media bizarrely conducted a coordinated attack on Oct. 12, 2021, when the U.S. Preventative Services Task Force reversed decades of protocol and recommended against using aspirin to prevent heart attacks and strokes. Suddenly, anything that may be used for COVID turns into poison overnight!
The media has also savagely attacked the use of betadine nasal rinse, which studies have shown has tremendous benefit for killing the viral load early on.
Thus, whether it’s broadly beneficial supplements and vitamins, cheap over-the-counter medications, safe prescription drugs, or experimental drugs used specifically for respiratory distress, nothing that actually might work need apply for approval. After all, they can only have an emergency if the public is kept in the dark on how to deal with it on their own.Never before have drugs as safe as hydroxychloroquine and ivermectin been deliberately demonized to the point that pharmacies refused to fill prescriptions. So countless Americans who were desperate for COVID treatment turned to Indian vendors for relief. Now, I’m getting numerous complaints from podcast listeners that they are having their packages confiscated by customs and the FDA. Meanwhile, Chinese fentanyl pours through the mail and other lethal drugs come over our border in plain sight, as even dark red states relax restrictions on marijuana. Welcome to a perfect morally bankrupt America.
Some of us never thought our government was capable of getting tough on border security, but one listener sent me a notice from the FDA’s Division of Northern Border Imports at a Chicago airport showing that her package is being held at customs. No, there are no illicit drugs. The package contained ivermectin, doxycycline, and zinc. All three are being held – even the zinc!
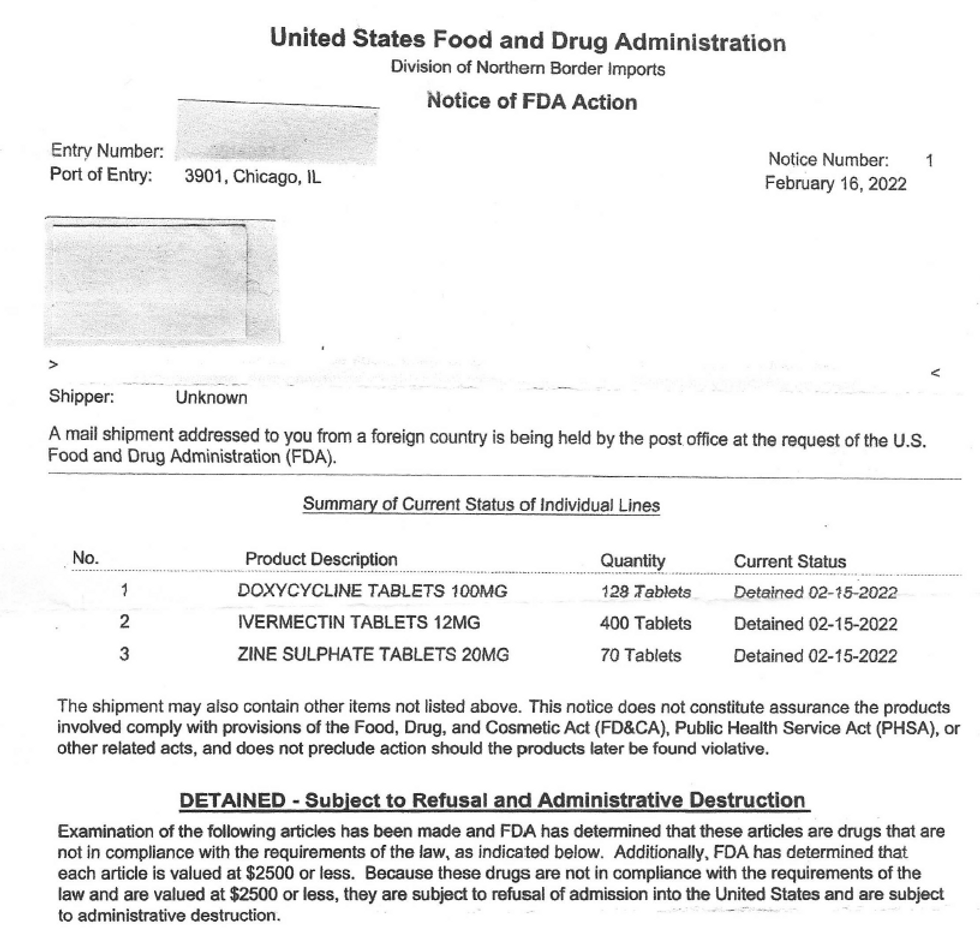
In the Notice of FDA Action, the inspection officials hide behind the fact that Section 801(a) of the FD&C Act gives them authority to go after products made by foreign facilities that aren’t proven to meet their standards. However, we all know that this has nothing to do with concern that somehow the products have manufacturing faults or impurities, but rather a back-door means of banning the FDA-approved ivermectin (and anything else that happens to work to treat COVID).
Federal courts have ruled in the past that the FDA can’t effectively use an import alert to change the rules of admissibility for a range of products without prior notice. For example, in 1992, the U.S. District Court for the Eastern District of New York ruled (Benten v. Kessler) that an import alert banning previously admissible abortifacient drugs constituted a substantive rule subject to notice-and-comment rulemaking. Although officials don’t claim to ban ivermectin in this notice, that is clearly their intent based on their laser-beam focus on this drug.
And speaking of enforcement priorities, perhaps their time would be better spent on searching imports of Chinese fentanyl, you know, the drug that is killing tens of thousands of people per year. The fentanyl comes in the mail and also comes from the Mexican cartels, which get shipments from China. The open-borders mass migration being induced by the Biden administration is the perfect ploy for the cartels to get their poison into our country. Isn’t that a more pressing issue?
It is shocking that government is attacking a drug that already is considered a safe, essential, and wonderous medicine, with FDA approval for years. It’s the last candidate to choose to begin an unprecedented and illegal war against long-standing medicines.
Once a drug is approved and particularly safe, we don’t even need data on efficacy as long as there’s informed consent. Even so, 53 studies from 48 independent teams in 22 different countries show statistically significant improvements in isolation against SARS-CoV-2. Even if one wants to quibble with relying on a few of them alone, the preponderance of evidence, in conjunction with understanding its 20 mechanisms of action against SARS-CoV-2, makes it incontrovertibly effective to some degree, and certainly in conjunction with other anti-inflammatories and supplements.
Just last week, University of Miami researchers published a retrospective study in the International Journal of Infectious Diseases of mortality outcomes among COVID patients treated with ivermectin versus those treated with remdesivir. The results showed a 69% reduction in mortality for those using ivermectin as compared to remdesivir, with a very high confidence interval. Incidentally, 69% is the exact reduction in mortality indicated in the Malaysian randomized controlled trial published in JAMA that Big Pharma tried to use to discredit ivermectin (by pursuing a random and impossible mid-level end-point). The outcome was slightly underpowered (at 91% confidence interval instead of the standard 95%), but again, the preponderance of evidence shows it works. And what about remdesivir? How is that even still on the market?
So, while ivermectin is treated worse than fentanyl, blocked at the ports, and shunned by pharmacies, Biden is now issuing an order that people can test for COVID and automatically get Pfizer’s and Merck’s drugs at the pharmacy. Let’s get this straight: One of the best drugs on the market with full approval for years is criminalized — with pharmacies having the ability to block it — yet two very new emergency-approval drugs with dangerous mechanisms of action, no independent proven efficacy beyond the manufactures’ own propaganda, and numerous contraindications with the very people for whom it is approved (high risk) can now be administered by a pharmacist — without a doctor? Our government has now made doctors into drug cartels and pharmacists into doctors, enabling Walgreens to block safe drugs and illegally administer dangerous and novel drugs without a doctor’s prescription!
There is no question that the war on off-label drugs is being waged by Big Pharma, which controls the global governments and the media. This news comes at a time when Dr. Tess Lawrie released a film showing how one researcher for the World Health Organization made a complete U-turn on support for ivermectin, leaving no doubt that strings are being pulled from the top.
What’s the GOP response? While there are numerous bills circulating in legislative bodies, very few of them have been signed into law. In South Dakota, with a 32-3 GOP majority in the Senate, a 62-8 majority in the house, and Kristi Noem as governor, the bill to ensure doctors can safely prescribe ivermectin was defeated. Yet South Dakota voters will have the opportunity to allow recreational marijuana in the state via a ballot referendum in November. Alabama is another allegedly conservative state that has allowed a recreational marijuana bill to pass out of a Senate committee, while completely failing to take action on a bill ensuring doctors are not punished for prescribing an FDA-approved drug.
The fact that the war on safe, effective, and established treatments is occurring precisely during the period of the most liberal deregulation of substances previously regarded as dangerous, as well as the expedited use of novel and emergency therapies, should tell you everything you need to know about the state of medicine in this country.The fights at the UFC Vegas 46 event had ended, but there were more clashes in the post-fight press conference on Saturday night. Dana White verbally sparred with a reporter who questioned the COVID-19 treatments that the UFC president took after contracting the respiratory disease.
During the post-fight press conference, White was questioned about his friend and UFC employee Joe Rogan. A reporter asked White about the group of 270 scientists, medical professionals, professors, and science communicators who signed an open letter demanding that Spotify adopt a policy on COVID-19 misinformation because they insisted that Rogan has a "concerning history of broadcasting misinformation, particularly regarding the COVID-19 pandemic."
White was not familiar with the open letter attempting to have Spotify censor Rogan over podcasts about COVID-19. White transitioned to COVID-19 treatments by saying, "Ever since I came out and said what I did, it's almost impossible now to get monoclonal antibodies."
The fully vaccinated White contracted COVID-19 during the Thanksgiving holiday, but was "feeling like a million bucks" in less than 24 hours after receiving COVID-19 treatments recommended by Rogan.
“I get up, 9 o’clock Monday morning, and I get tested. He said get monoclonal antibodies in you as soon as possible, so I did," White told sports podcast host Jim Rome on Dec. 1. "By noon, I had the monoclonal antibodies in me. Then he told me to do a NAD drip. I did that right after."
White added that he had a dose of ivermectin and a vitamin drip.
During Saturday's press conference, White alleged, "They're making it so you can't get them. Medicine that absolutely works — they're keeping from us."
The UFC president claimed, "I don't want to get too political and start getting into all this sh**, but ivermectin and monoclonal antibodies have been around for a long time. Now all of a sudden you can't dig them up to save your life; the doctors won't give them to you."
White said that he "made one phone call" and was able to get monoclonal antibody treatment.
"And that's not some f***ing rich famous guy sh**," White added. "Like anybody could've called...Everybody could've got it back then."
He then claimed, "You can't get those things to save your life now, literally."
In late December, the federal government halted the distribution of two of the three monoclonal antibody treatments, claiming they were ineffective against the Omicron variant. According to the FDA, only the monoclonal antibody treatment called "sotrovimab" by GlaxoSmithKline and Vir Biotechnology is effective against Omicron. However, it is in short supply.
White called the lack of COVID-19 therapeutics "disgusting" and "one of the craziest things that I've ever witnessed in my life."
"And we're not talking about experimental drugs or things," White continued. "This stuff's been around. Ivermectin, the guy won a Nobel Peace Prize."
Then Yahoo reporter Kevin Iole interjected, "Are you a doctor?"
White replied, "No, but I took them, and they both worked for me, so why shouldn't I be able to take them again? Or other people?"
White roared back, "You want to know what's scary? I bet I can get some f***ing pain pills quicker than I can get monoclonal antibodies. Not maybe; that's a fact. They f***ing hand out pain pills like they're Tic Tacs. Pain pills kill you. Fact. And I’m not a doctor, but that’s a fact."
Content Warning: Strong language:
UFC President Dana White is asked about 200+ Doctors demanding Spotify censor Joe Rogan.pic.twitter.com/hNrJ6UVHmK— The Post Millennial (@The Post Millennial) 1642356215
For Americans suffering from COVID-19 and seeking life-saving treatment, they may find themselves put on a waitlist depending on their skin color. The Food and Drug Administration, as well as multiple states, will prioritize race and ethnicity in determining who receives hard-to-get COVID-19 therapeutics. In some cases, even those with dangerous health conditions could be passed over for someone who is "non-white."
GlaxoSmithKline's sotrovimab is reportedly the only monoclonal antibody treatment that is effective against the Omicron variant — however, it is in very short supply.
The FDA's "fact sheet" for sotrovimab presents health care providers with a guidance on the emergency use authorization of the treatment. There is a "patient selection" section that outlines who should receive the sotrovimab treatment, especially since it is in limited availability at this time. The fact sheet lists "medical conditions or other factors may place adults and pediatric patients at higher risk for progression to severe COVID-19." Some of the conditions on the list include being aged 65 or older, obesity, chronic kidney disease, diabetes, and cardiovascular disease.
The guidance also adds a stipulation:
Other medical conditions or factors (for example, race or ethnicity) may also place individual patients at high risk for progression to severe COVID-19, and authorization of sotrovimab under the EUA is not limited to the medical conditions or factors listed above.
The Centers for Disease Control and Prevention states that high-risk individuals include people over age 65 and those with underlying medical conditions.
The CDC also proclaims:
Long-standing systemic health and social inequities have put various groups of people at increased risk of getting sick and dying from COVID-19, including many people from certain racial and ethnic minority groups and people with disabilities.
Studies have shown people from racial and ethnic minority groups are also dying from COVID-19 at younger ages. People in minority groups are often younger when they develop chronic medical conditions and may be more likely to have more than one condition.
Some states have adopted the guidances from the FDA and CDC that prioritizes race or ethnicity when determining who receives the therapeutic.
Last month, Minnesota released the state's "Ethical Framework for Allocation of Monoclonal Antibodies during the COVID-19 Pandemic" guidance.
The FDA has acknowledged that in addition to certain underlying health conditions, race and ethnicity "may also place individual patients at high risk for progression to severe COVID-19.' FDA’s acknowledgment means that race and ethnicity alone, apart from other underlying health conditions, may be considered in determining eligibility for mAbs. It is ethically appropriate to consider race and ethnicity in mAb eligibility decisions when data show elevated risk of poor COVID-19 outcomes for Black, Indigenous and other people of color (BIPOC populations), and that this risk cannot be adequately addressed by determining eligibility based on underlying health conditions (perhaps due to underdiagnosis of health conditions that elevate risk of poor COVID-19 outcomes in these populations).
The guidance adds, "At the present time, MDH has found that available data show this elevated risk."
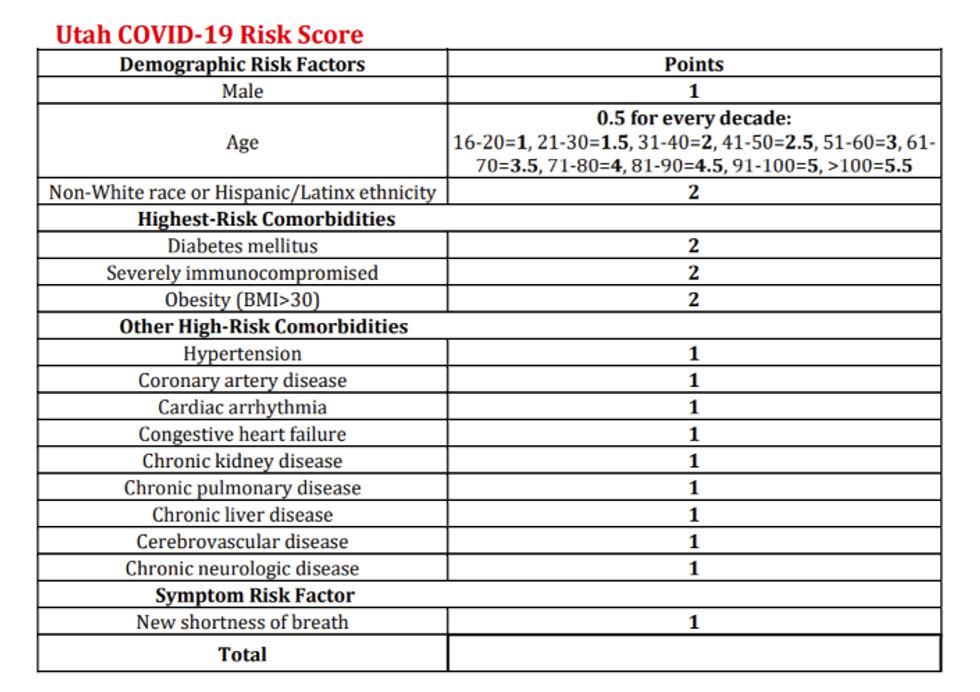
In Utah, the state released its "Crisis Standards of Care Monoclonal Antibody Allocation Guidelines."
The guideline provides a risk score based on risk factors such as age and obesity and assigns a point value to each factor. Shockingly, being "Non-White race or Hispanic/Latinx ethnicity" holds a higher risk value than high-risk co-morbidities such as hypertension, coronary artery disease, cardiac arrhythmia, congestive heart failure, chronic kidney disease, chronic pulmonary disease, chronic liver disease, cerebrovascular disease, chronic neurologic disease, and those suffering from shortness of breath.
Earlier this month, TheBlaze reported on the New York state government's decision to prioritize non-white people to receive COVID-19 treatments because of "longstanding systemic health and social inequities."
When it comes to the distribution of monoclonal antibodies as well as the new oral antiviral pills — Pfizer's Paxlovid and Merck's molnupiravir — New York's Department of Health declares, "Non-white race or Hispanic/Latino ethnicity should be considered a risk factor, as longstanding systemic health and social inequities have contributed to an increased risk of severe illness and death from COVID-19."
As COVID-19 rips through New York, the state government has decided to prioritize non-white people to receive COVID-19 treatments because of "longstanding systemic health and social inequities."
Last week, New York’s Department of Health released a document titled: "Prioritization of Anti-SARS-CoV-2 Monoclonal Antibodies and Oral Antivirals for the Treatment of COVID-19 During Times of Resource Limitations Introduction." The memo provides a hierarchy of who should receive the limited supplies of monoclonal antibodies as well as the new oral antiviral pills — Pfizer's Paxlovid and Merck's molnupiravir — that were approved by the U.S. Food and Drug Administration last week under Emergency Use Authorization.
New York state offers a framework on which individuals should receive priority for COVID-19 treatments based on "risk factors," which include those who are immunocompromised, those who are aged 65 or older, and those who are overweight.
There is also a "note" that states that any COVID-infected people who are non-white should receive priority for treatment over white people because of "inequities."
"Non-white race or Hispanic/Latino ethnicity should be considered a risk factor, as longstanding systemic health and social inequities have contributed to an increased risk of severe illness and death from COVID-19," New York’s Department of Health states.
NY State Department of Health warns they don't have enough Paxlovid or Monoclonal Antibody Treatment and white people need not apply. http://www.mssnyenews.org/wp-content/uploads/2021/12/122821_Notification_107774.pdf\u00a0\u2026pic.twitter.com/MwBtjv2pDx— Karol Markowicz (@Karol Markowicz) 1640916049
Erin Silk, a spokesperson for the New York Department of Health, told Fox News, "Systemic poverty, which has clearly proven to be a risk factor in populations in New York State and nationwide, is added to the algorithm of prioritization similar to all other risk factors. It is merely mentioned as a factor that increases risk."
The spokesperson noted that the state's "prioritization guidance comes directly from the CDC," adding "Race nor ethnicity would disqualify an individual from receiving treatment."
The Centers for Disease Control and Prevention states, "Race and ethnicity are risk markers for other underlying conditions that affect health, including socioeconomic status, access to health care, and exposure to the virus related to occupation, e.g., frontline, essential, and critical infrastructure workers."
The New York City Department of Health also recommends that race should be considered when prioritizing who receives monoclonal antibodies and oral antiviral pills for COVID-19.
"Consider race and ethnicity when assessing individual risk, as longstanding systemic health and social inequities may contribute to an increased risk of getting sick and dying from COVID-19," the memo to providers reads.
In May, Dr. Anthony Fauci claimed that the COVID-19 pandemic exposed "the undeniable effects of racism" in America.
"Now, very few of these comorbidities have racial determinants," said the chief medical adviser to President Joe Biden. "Almost all relate to the social determinants of health dating back to disadvantageous conditions that some people of color find themselves in from birth regarding the availability of an adequate diet, access to health care, and the undeniable effects of racism in our society."
Dana White revealed he contracted COVID-19 during the Thanksgiving holiday, but he is already feeling better after being administered the same coronavirus treatment that Joe Rogan took when he was infected.
The UFC president appeared on "The Jim Rome Podcast" on Wednesday, where he provided a health update. White believes he contracted COVID-19 after going to his family's Thanksgiving celebration in Maine.
"We just went up to my place in Maine, and for Thanksgiving, it’s tradition, we go up there, and somebody had it, and we get back, and we all tested positive for COVID," White told host Jim Rome. "Literally the whole family and my family up in Maine, too."
White returned home on Saturday, but on Sunday, he noticed that he didn't have his sense of smell – a symptom of the illness.
"I cold plunge and steam every day," White explains. "So I get out of the cold plunge and steam, and I spray the eucalyptus and I was like, 'What the hell?' I couldn’t smell anything. So I opened the bottle and start sniffing the bottle of eucalyptus, and I'm like, 'Yeah, I got no smell.' I said, 'You know what this means.'"
"I literally got out of the steam and got on my phone and called Joe Rogan," White said.
“I get up, 9 o’clock Monday morning, and I get tested. He said get monoclonal antibodies in you as soon as possible, so I did," White said of Rogan's advice. "By noon, I had the monoclonal antibodies in me. Then he told me to do a NAD drip. I did that right after."
"I get up Tuesday getting ready to shave," he continued. "Cleaning my razor, I could smell the alcohol. My taste and smell were back by the next day by 11 o’clock. Then I took a dose of ivermectin. Then yesterday I did a vitamin drip, and today I’m doing another NAD drip."
"Could not feel better. I’m feeling like a million bucks," White exclaimed. "I’m doing two-a-day workouts for the next 10 days while I have COVID and I’m in quarantine. I got my smell and taste back in less than 24 hours."
Rome asked White, 52, why he consulted Rogan first before a doctor.
"Listen, I’m vaccinated," White responded. "It’s not like I’m some crazy anti-vax conspiracy theorist or some of that stuff but Rogan is a very brilliant guy. Very smart guy who talks to the best and the brightest out there, and I’m not a believer in the narrative."
"But at the end of the day, this is a free country. Cause what happens when you get this stuff, they tell you stay home for the next 10 days until you don’t test positive. That doesn’t seem smart to me. Just like when we went through COVID, I believe in finding solutions to problems and answers.”
"Rogan has worked with over 30 or 40 people that have done this and he swears by it, and he’s a good friend of mine that I’ve known for over 20 years. So yeah, I believe in what he’s saying. I believe the things he explained to me on how this thing works made sense to me," White said.
"If you got COVID, I would urge you to do this," White told Rome. "Not only did I believe in it before I did it, now I've actually done it. I'm on day three of COVID. Day three of COVID, smell and taste is back. I could not feel better and I'm doing two-a-day workouts while I'm going through COVID."
"I’m attacking this thing with the methods that Rogan has learned from some very smart people," he added. "I’m going to keep testing every two days until I’m negative and then I’m going to get back to work ASAP."
White said he and his family are currently in isolation. He hopes to attend the Ultimate Fighting Championship's next event on Saturday – UFC Vegas 44 at the UFC Apex in Las Vegas, Nevada.
"If I test negative, then absolutely, positively I’ll be there," he said. "I’m going to do the exact protocol that’s supposed to be done to make sure I’m clean and can go and be around people again. As soon as that’s 100% clear, then I’ll be back to work."
Previously, All-Pro Green Bay Packers quarterback Aaron Rodgers consulted Rogan on COVID-19 treatments after he tested positive for coronavirus. After subscribing to the same treatment that Rogan was prescribed, Rodgers was activated off the reserve/COVID-19 list, just 10 days after he tested positive for coronavirus — the earliest he was eligible to return.
On Oct. 28, the unvaccinated Rogan tested positive for COVID-19 and "immediately threw the kitchen sink at it." His treatment consisted of "all kinds of meds, monoclonal antibodies, ivermectin, Z-Pak, prednisone, everything."
By Sept. 1, he said, "I actually feel pretty f***ing good."
On Sept. 3, Rogan, 54, tested negative for COVID-19.
First it was lockdowns, then masks, then a series of failed shots and boosters, with a side-dish of remdesivir killing people in hospitals while blocking lifesaving treatments. Now, the same forces within big pharma are about to unleash their first outpatient drug on us. Are we really to believe that this will suddenly be the first pristine COVID product from big pharma that actually helps rather than harms us? Color me skeptical, especially based on what we know about Merck's supposed wonder drug, Molnupiravir.
First, it's important to note that the medical establishment has made it clear they will not touch a repurposed drug so long as it's off-patent. However, they have no problem using repurposed drugs that are expensive and unsafe as they did with remdesivir (which was repurposed from the Ebola virus) after it killed so many people and had to be pulled from trial. Well, Molnupiravir is also a repurposed drug. Wait for it … Molnupiravir is actually repurposed from a horse drug!
Merck's Molnupiravir, also known as (EIDD-2801), was originally co-developed by Ridgeback Biotherapeutics LP and a biotech company owned by Emory University in 2003 to treat equine encephalitis and was later purchased by Merck to be used for coronavirus. There is nothing novel about the drug. It is a nucleotide analogue that introduces errors in the viral RNAat the time of replication after to cause mutations. The problem with mutagenic drugs is that they are known both to cause side effects, such as cancer and birth defects, in the individual, as well as spawn mutations in the virus — not coincidently similar to the leaky vaccines currently being used.
The reason drugs like ivermectin that work "accidentally" against viruses are so much safer is precisely because they don't attempt to go after the virus in an offensive way, running the risk of damaging good cells and causing mutations. It's actually a good thing that they are not traditional anti-virals and work in a defensive way. The reason we have so few anti-virals in the first place is because they are dangerous to the patient, sort of like chemotherapy.
Merck is asking for federal emergency use authorization at the same time the federal government has signed a $1.2 billion contract with them based on a single safety study conducted by ... you guessed it ... the very entity itself. Interestingly enough, the study participants were bizarrely told to abstain from "heterosexual intercourse" during the trial, a rare and interesting distinction for a trial protocol in an anti-viral therapeutic. But, in fact, they understood that this technology, which works similar to chemotherapy, is not safe for women of childbearing age who want to have children in the future. As Dr. Simon Clarke, associate professor in cellular microbiology at the University of Reading, said, the specific instructions on heterosexual sex "suggests that the drug has the potential to cause birth defects should someone become pregnant."
Much like vaccines, anti-viral agents have to be formulated perfectly. If they fail to fully kill the virus, they run the risk of having the virus mutate and becoming more virulent, akin to shooting at the king and missing.
Also, in the same way anti-virals attack the genetic makeup of the viruses, they have the potential to damage the hosts as well, just like we see with anti-cancer drugs. "Proceed with caution and at your own peril," wrote Raymond Schinazi, a professor of pediatrics and the director of the division of biochemical pharmacology at the Emory University School of Medicine, who has studied NHC for decades, in an email to "Barron's" last month.
After the body ingests Molnupiravir, it produces a compound called NHC (N-hydroxycytidine). Schinazi, who works at the university that originally developed the drug, co-authored a paper in the Journal of Infectious Diseases a few months ago warning that NHC caused mutations in animal cell cultures. Schinazi used to own a biotech company that once attempted to pursue this drug, but abandoned it in 2003 once it was discovered it's mutagenic tendencies.
Barron's also interviewed Dr. Shuntai Zhou, another author of the study and a scientist at the Swanstrom Lab at UNC, who claims he warned Merck last year about their initial findings.
"There is a concern that this will cause long-term mutation effects, even cancer," Zhou says.
Zhou says that he is certain that the drug will integrate itself into the DNA of mammalian hosts. "Biochemistry won't lie," he says. "This drug will be incorporated in the DNA."
What impact it will have when it's there is unknown, given the various systems human cells use to limit the impact of mutations.
Well, that sounds like a drug we want to dive into headfirst while we have safe, cheap, and effective alternatives.
Merck disputes these claims and suggest their studies show otherwise. But are we really to once again place our blind trust in a company that stands to make billions when known molecular biological principles should caution us against proceeding forward with this technology? Why would we spend billions on an untested drug with a dangerous mechanism of action when safe, off-patent, repurposed drugs already work better without the risk?
In another violation of informed consent, Merck was given $356 million in taxpayer funding at the beginning of the pandemic to develop this already-developed drug, despite opposition at the time from former director of the Biomedical Advanced Research and Development Authority (BARDA) Rick Bright. He warned that "similar experimental drugs in this class had been shown to cause reproductive toxicity in animals, and offspring from treated animals had been born without teeth and without parts of their skulls."
It is simply indefensible to pursue a drug like this when we already have established, effective, less expensive drugs that are actually safe. Even on side of efficacy, it's becoming harder to take Merck's press released "study" at face value. Two Indian companies that contracted with Merck have already pulled out of the deal because they believe it has no efficacy against moderate COVID-19 infections. Merck had already suspended its trials on Molnupiravir for hospitalized COVID patients, but in October, Aurobindo Pharma Ltd and MSN Laboratories pulled their studies after the drug failed to yield positive results with those who already had a moderate version of the virus.
The fact that Merck is seeking approval to make this drug a standard of care after their trial only followed up on safety data for 29 days in the human trials is unprecedented. The results of their human "trial" were never published in a peer-reviewed journal, the same as a press release released only on their website. "Merck says" their drug is safe and effective is in line not just for approval in the free market, but for government purchases of over a billion dollars' worth of doses.
In the preprinted results of the study by Ridgeback employees last December, they made mention of toxicity in the bone marrow found in the dogs that were part of the animal trial. Interestingly enough, when the study was published in an actual journal several months later, the reference to toxicity in the bone marrow disappeared. However, in the approval paper from the U.K.'s Medicines and Healthcare Products Regulator Agency, they mention, "Reversible, dose-related bone marrow toxicity affecting all haematopoietic cell lines was observed in dogs at ≥17 mg/kg/day (0.4 times the human NHC exposure at the recommended human dose (RHD))." That is not a large enough dose to achieve toxicity.
Shockingly, the U.K. regulator also concedes that "Carcinogenicity studies with molnupiravir have not been conducted," even though long term cancer risks are always a concern with nucleotide analogues. The agency also revealed that "No clinical interaction studies have been performed with molnupiravir."
Sadly, from everything we have seen with the shots and with remdesivir, the less safety data that is available, the more likely the FDA is to approve the drug — in violation of 100 years of standard protocols for drug approval.