'Eat real food': Trump administration flips 'corrupt food pyramid,' encourages meat and veggies over bread and oatmeal

In the ongoing effort to make America healthy again, Health and Human Services Secretary Robert F. Kennedy Jr. and other members of the Trump administration gathered for a special press conference on Wednesday to announce a major overhaul of dietary guidelines
The guidelines, promoted under the simple command to "eat real food," introduce a "new pyramid" that prioritizes protein, dairy, healthy fats, and fruits and vegetables over whole grains, which is essentially an upside-down version of the conventional food pyramid most people are familiar with.
'These guidelines replace corporate-driven assumptions with common-sense goals and gold-standard scientific integrity.'
"These guidelines replace corporate-driven assumptions with common-sense goals and gold-standard scientific integrity," Kennedy said at the press conference.
He added that they will "revolutionize our nation's food culture and make America healthy again."
RELATED: Trump administration overhauls childhood vax schedule. Here's the downsized version

“For decades, we’ve been fed a corrupt food pyramid that has had a myopic focus on demonizing natural healthy saturated fats, telling you not to eat eggs and steak, and ignoring a giant blind spot: refined carbohydrates, added sugars, ultra-processed food,” Food and Drug Administration Commissioner Dr. Marty Makary said.
Secretary of Agriculture Brooke Rollins said, "A healthy meal is within reach for all American families. These new dietary guidelines are a framework which is meant to be customized to meet the needs, the preferences, and the financial status of all American families."
The inverted pyramid is the result of many studies conducted by the government to challenge the current paradigm and address our nation's health problems. The guidelines were published in multiple documents, including a series of appendices that is over 400 pages long.
Some users on social media joked that HHS was copying a "South Park" bit in which scientists, at the behest of character Eric Cartman, "flip the pyramid" to reveal the "true" nutritional standards.
The old food pyramid originated in Sweden in the 1970s and was later adapted by the United States Department of Agriculture in 1992.
Like Blaze News? Bypass the censors, sign up for our newsletters, and get stories like this direct to your inbox. Sign up here!
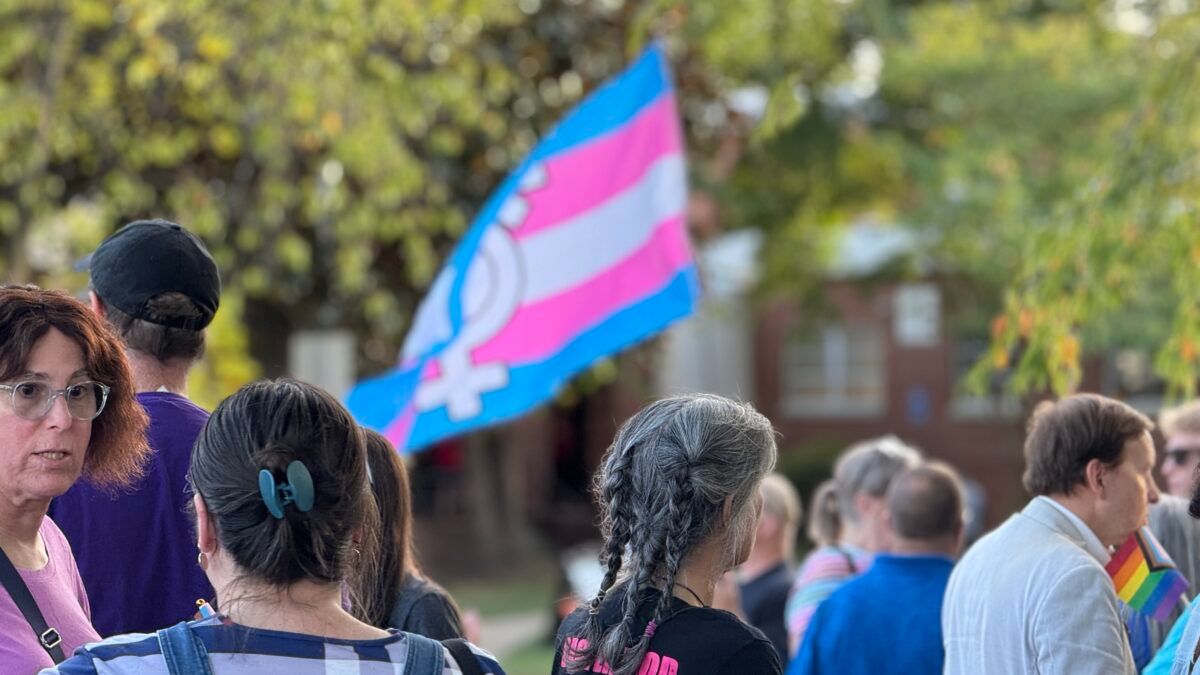 The Department of Health and Human Services (HHS) announced Thursday morning that it will be pursuing several measures to hold states, hospitals, and companies accountable for pushing harmful “gender transition” on children. On a call with members of the media, an HHS official from the Centers for Medicare and Medicaid Services (CMS) said the agency […]
The Department of Health and Human Services (HHS) announced Thursday morning that it will be pursuing several measures to hold states, hospitals, and companies accountable for pushing harmful “gender transition” on children. On a call with members of the media, an HHS official from the Centers for Medicare and Medicaid Services (CMS) said the agency […] Widespread support for having kids and raising a family is exactly why the Hawleys say they created Love Life Initiative.
Widespread support for having kids and raising a family is exactly why the Hawleys say they created Love Life Initiative.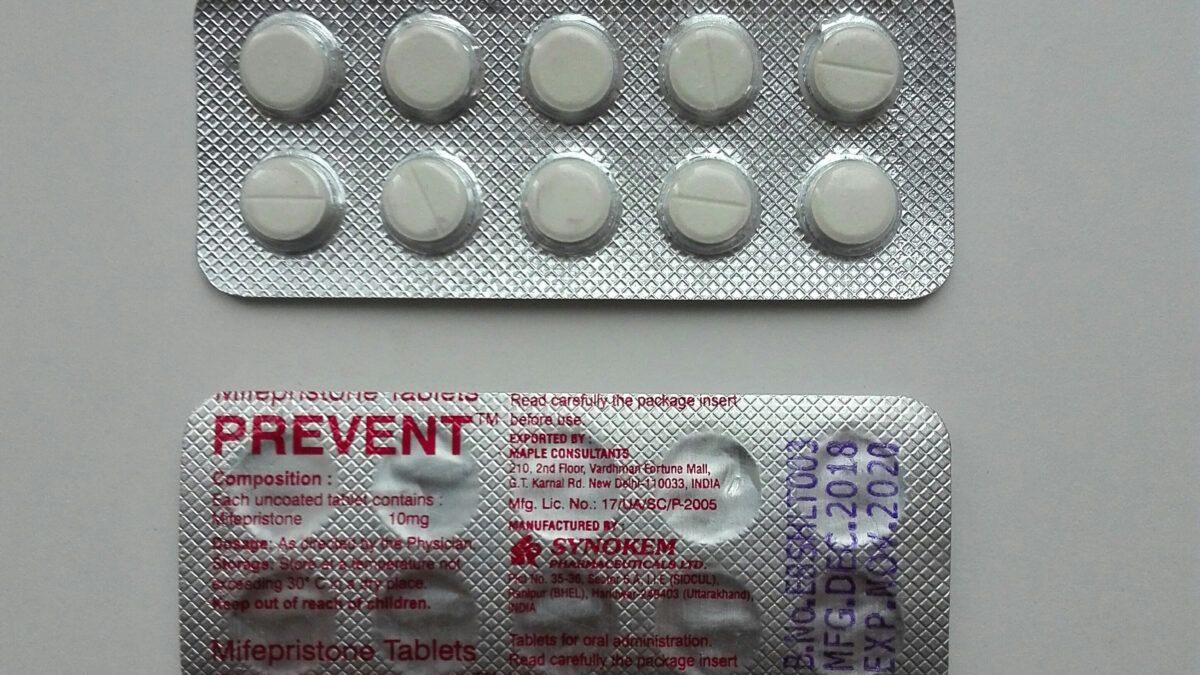 The medical reasons that led prior FDAs to require in-person doctor visits before women could access the abortion pill still stand.
The medical reasons that led prior FDAs to require in-person doctor visits before women could access the abortion pill still stand.
 The FDA’s study, Makary announced, is even ‘bigger’ and ‘more robust’ than previous mifepristone examinations.
The FDA’s study, Makary announced, is even ‘bigger’ and ‘more robust’ than previous mifepristone examinations. American teenagers have a fractured relationship with the federal government, one that may never be healed.
American teenagers have a fractured relationship with the federal government, one that may never be healed.
 Photographer: Daniel Acker/Bloomberg via Getty Images
Photographer: Daniel Acker/Bloomberg via Getty Images
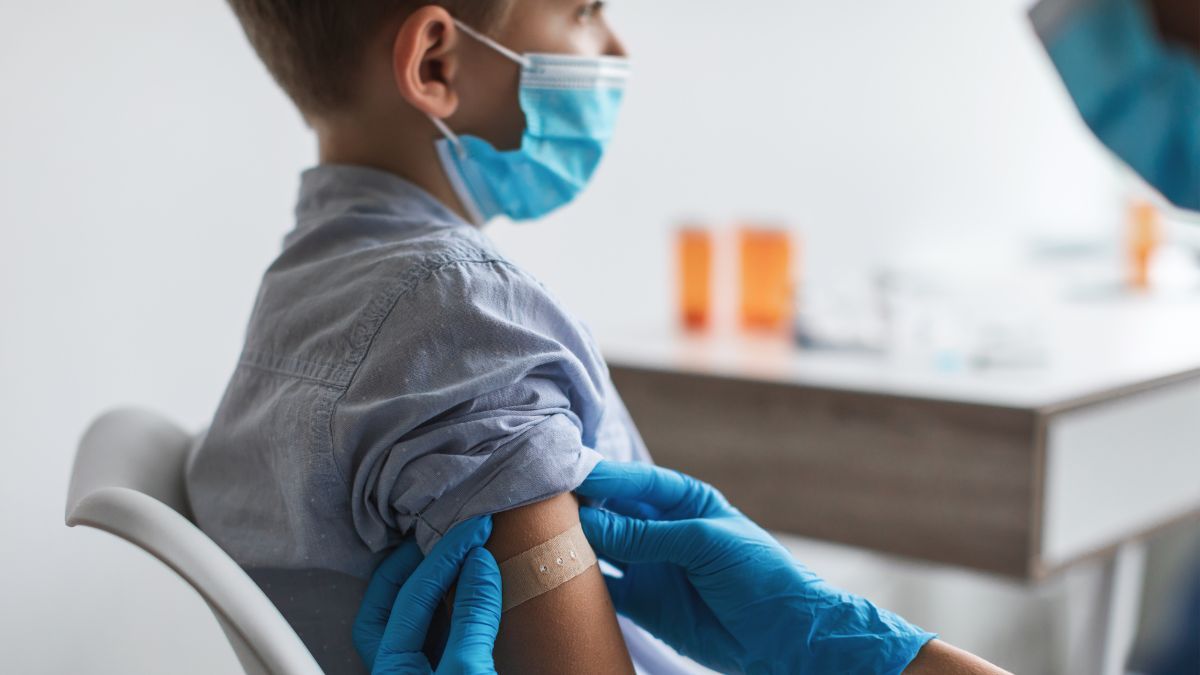 'Like many academic physicians, we felt the FDA and CDC abdicated their duty to the American people,' Prasad wrote.
'Like many academic physicians, we felt the FDA and CDC abdicated their duty to the American people,' Prasad wrote.