COVID 2.0? New RSV shots are already harming babies

I might not be a doctor, but as early as January 2022 — 16 months before the approval of the first RSV vaccine — I warned that these shots could make children sicker from the virus. For decades, the industry failed to produce an RSV vaccine after an attempt in 1967 was terminated because it caused antibody-dependent disease enhancement.
Now, amid ongoing problems with the first RSV vaccines from Pfizer and GSK, the FDA is acknowledging that Moderna’s mRNA version is causing severe RSV cases in children. It’s time for the incoming Department of Health and Human Services, along with state officials, to pull the plug on both RSV shots and mRNA vaccines of all kinds.
We have simply too much public information to sustain this deception any longer. The time for action on this long-standing failure is January 20, not a day later.
The FDA reported last week at least five cases of severe or very severe RSV in infants who received Moderna’s new mRNA vaccine during a clinical trial. The Biden administration had already approved Pfizer's and GSK’s senior RSV vaccines, Pfizer’s infant vaccine, and a monoclonal antibody treatment for young children — all of which have documented safety concerns. Earlier this year, the administration approved Moderna’s mRNA version (mRESVIA) for seniors.
However, the FDA has now revealed that enrollment of young children in clinical trials is “on hold for all clinical studies of RSV vaccine candidates” under U.S. investigational new drug protocols.
The clinical trial showed shocking results: “Severe illness was 26.3% in the vaccine groups compared with 8.3% in the placebo.” Talk about “the more you inject, the more you infect”! If there are so many problems with infants, why are we giving this shot to anyone — especially seniors, who are not at significant risk for RSV the way they are for flu or COVID?
Moderna used two versions of the vaccine in the clinical trial, and one of them, mRNA-1345 (mRESVIA), induced severe RSV in an infant. This same vaccine is now being administered to seniors.
Given the 60-year concern about enhanced disease, why are we continuing to push any RSV shots? Why are we continuing to approve mRNA vaccines after the documented issues with COVID shots? Why approve Pfizer’s RSV shot for pregnant women when GSK’s nearly identical version was pulled from clinical trials after causing death and injury?
Finally, why are we promoting vaccines for respiratory viruses at all? COVID and flu have shown that respiratory viruses do not respond well to blood-based antibodies and often cause immune imprinting, leading to a higher risk of infection in the long run.
These are straightforward questions any layman can understand, yet our medical experts remain stuck in ignorance — and greed.
Greater risk for babies
Pfizer's and Moderna’s clinical trials for the COVID vaccine in toddlers revealed that leaky respiratory viral vaccines tend to increase, not decrease, the risk of respiratory viruses. In Moderna’s trial of babies ages 6 to 23 months, researchers found a statistically significant increase in respiratory viruses within 28 days of vaccination.
For croup, 1.3% of mRNA-1273 recipients were infected, compared to just 0.3% of placebo recipients. For RSV, the infection rate was 0.8% for mRNA-1273 recipients and 0.5% for the placebo group. For pneumonia, 0.2% of mRNA-1273 recipients were affected, while no cases occurred in the placebo group.
In Moderna’s trial for children ages 2 to 5 years, 0.3% of participants developed pneumonia compared to none of the placebo recipients. For RSV, the rates were 0.4% for vaccinated children and less than 0.1% for the placebo group. In other words, young children in the vaccine group were four times more likely to contract RSV within four weeks of the shot than those in the placebo group.
This trend persisted even among 6- to 11-year-olds, who are less susceptible to RSV. In that group, 0.3% of vaccine recipients experienced the illness compared to zero cases in the placebo group.
Pfizer’s children’s vaccine clinical trial for toddlers (see page 51) also recorded serious adverse events, including RSV bronchiolitis (five participants), pneumonia (two participants), gastroenteritis (two participants), and lower respiratory tract infections (two participants).
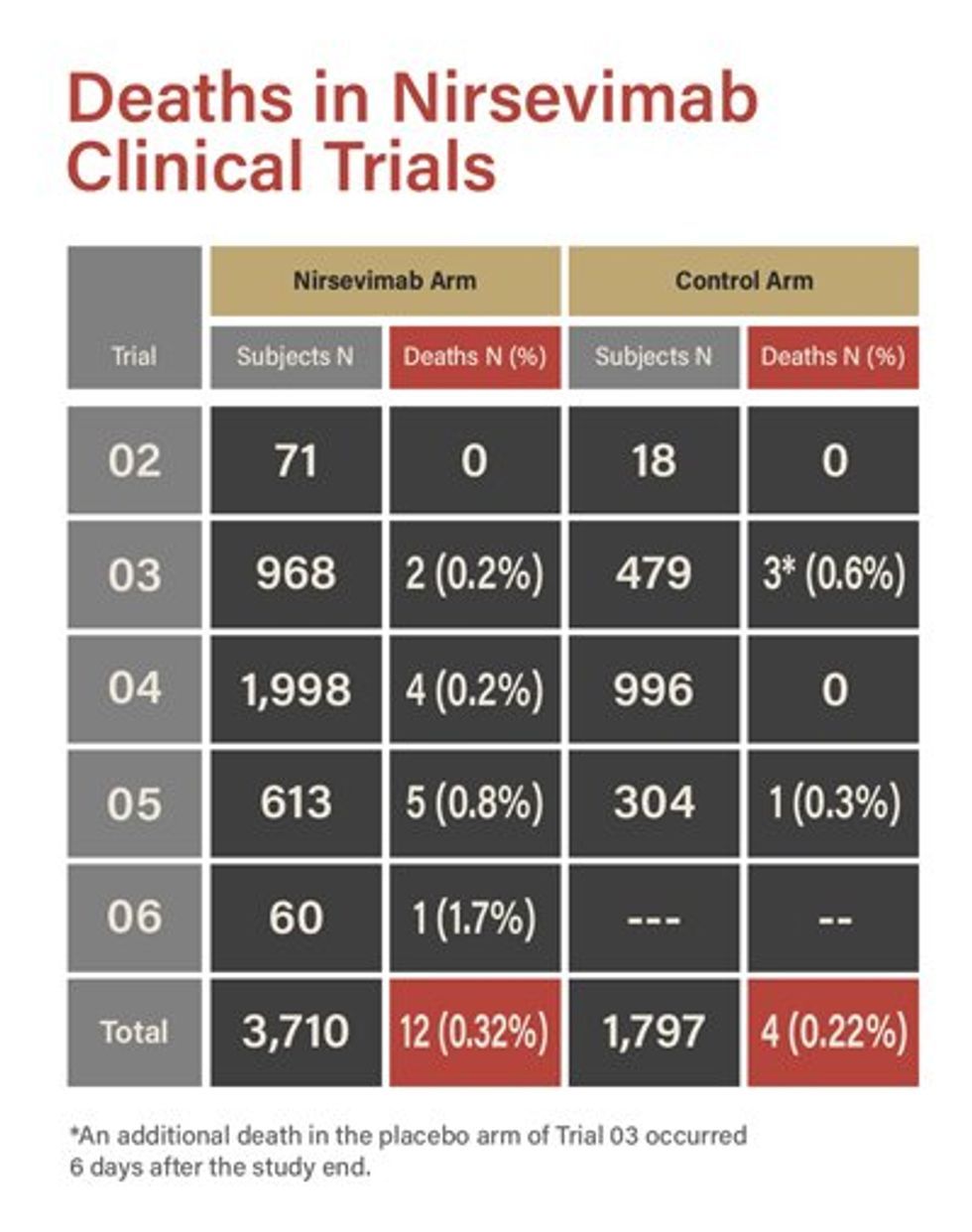
Clearly, respiratory viral vaccines make individuals more vulnerable to RSV. Many people now avoid these vaccines, but the industry has adopted a clever marketing tactic: offering a monoclonal antibody as a prophylactic measure against RSV, alongside the GSK, Pfizer, and Moderna shots. Originally developed by AstraZeneca and now distributed by Sanofi, Beyfortus (nirsevimab) has been administered to newborns since last October. New mothers are convinced their babies could suddenly die from RSV, which may have originated in the 1950s through polio vaccine research. According to the CDC, 40.5% of babies in America received Beyfortus during the last RSV season.
The FDA’s briefing document on Moderna’s clinical trial reported that the Moderna shot not only worsened the disease but also “blunted” Beyfortus’ efficacy in babies who received both. But what the report fails to disclose is that Beyfortus is as problematic as Moderna’s mRESVIA.
After hundreds of thousands of French babies received Beyfortus in 2023, Dr. Helen Banoun identified a shocking and unexplained increase in infant deaths linked to the vaccine’s uptake. Banoun also highlighted the FDA’s own data, which shows — clearly and alarmingly — a much higher rate of deaths in the Beyfortus treatment group compared to the placebo. This data appears on page 70 of the Biologics License Application for Beyfortus, but it seems the manufacturers rely on no one reading it.
Other literature cited in the Banoun paper shows that children were getting sicker with respiratory illnesses, signaling immune imprinting and disease enhancement — negative efficacy against the very illness the vaccines are supposed to treat.
According to VAERS reports, a baby boy died immediately after receiving the injection, and a baby girl was found unresponsive seven hours later. “Sudden infant death syndrome,” indeed.
What Trump can do
Moreover, the problem extends beyond the individuals receiving the vaccine. Dr. Peter McCullough has documented growing evidence that these products may be creating super-resistant strains of RSV.
We cannot continue like this as a civilization. Continuing mass vaccination without immediate restrictions and research violates the principles of the pro-life movement, based on the available data. It also violates the Nuremberg Code on human experimentation.
To that end, Trump’s next HHS secretary would do well to implement the following policies regarding vaccines:
- No shot should be marketed as a vaccine in any way if it is not proven to stop infection.
- No shot should be approved without a full placebo group that is kept permanently to study long-term differences with the trial group.
- No shot should be approved unless it shows an all-cause mortality benefit over time and most certainly cannot show more deaths in the trial group.
- No shot should be approved for one age group when there are clear safety signals in other age groups, unless it can be proven that those safety issues do not apply to the targeted cohort. For example, even after the FDA admitted that RSV shots caused Guillain-Barré syndrome and walked back its approval for people over 60, the agency still recommends the shots for people over 75.
- No mRNA shots whatsoever.
- No shot should be approved without oncogenicity, genotoxicity, or long-term safety studies, none of which were conducted with any of the RSV shots.
This issue is no longer just about COVID or Operation Warp Speed. The RSV vaccine approvals were conducted openly, despite known problems with these shots from day one — just like in 1967. They didn’t even need to rely on emergency use authorization.
We have simply too much public information to sustain this deception any longer. The time for action on this long-standing failure is January 20, not a day later.