Horowitz: Paxlovid and the need to end human experimentation
The more you “pax,” the more you vaxx.
You have to hand it to Pfizer. The company concocted a brilliant marketing strategy for two products that self-fulfill a need for each other. The more you vaxx, the more likely you are to get COVID. Then they offer the therapeutic Paxlovid upon getting the virus, in which case you get a rebound of the virus and take even more Paxlovid. Then, because of the rebound, you are so scared that you get more boosters, which of course, erase your natural immunity and make it even more likely that you get the virus again … and need Pfizer’s pair of products a second time. Rinse and repeat while Pfizer’s Albert Bourla and his FDA and NIH cronies laugh all the way to the bank.
What is the statistical likelihood that the president, the first lady, the CDC director, and Dr. Fauci all got the virus shortly after their booster shots and all got a rebound of the virus after taking Paxlovid – if it’s really a rare phenomenon? On Monday, CDC Director Rochelle Walensky announced that three weeks after getting her fifth COVID shot in less than two years and contracting COVID nonetheless, and a week after taking Paxlovid, she got the mysterious “Paxlovid rebound” and tested positive again. How can the government continue to support, market, and endorse both of these products after all of the known and unknown problems – after the president himself conceded the emergency is over?
Consider the following: Paxlovid is an experimental drug mixed with a heavy-duty AIDS drug with over 30 categories of contraindications with common medicines that most seniors take. It clearly causes a rebound of the virus, which is already mild to begin with for the Omicron variants, and leaves people with a metallic taste in their mouths. No long-term genotoxicity and carcinogenicity studies were conducted, and a new study shows that it might cause blood clots, just like the Pfizer shots. Yet this drug is being funded by the government and now being dispensed at pharmacies without a doctor’s prescription, while safe, Nobel Prize-winning drugs are being denied by pharmacists even with a doctor’s prescription!
But they are not done yet. Now, the NIH is rewarding Pfizer’s executives for their malfeasance by studying Paxlovid as the first candidate for “long COVID treatment.” This is the brilliance of their marketing at work. If their products actually worked, they would only make a finite amount of money. But with their products causing counterproductive reactions and relapses of the virus, they create a perpetual circuitous cycle and pretext to use them over and over again at “the speed of science.” Paxlovid has brought in $7.5 billion in sales just for Q3 alone and $22 billion for the year, a pretty good return for the greatest rebounder since Dennis Rodman.
This farce is no longer funny any more. It’s no longer enough for Republicans to merely inveigh against mandates while asserting the shots don’t stop transmission. The shots and Paxlovid actually have negative efficacy and many known and unknown side effects. They need to be immediately pulled from the market, the entire approval process needs to be investigated and overhauled, and legislation needs to be established to prevent this from ever happening again, including provisions limiting liability protections and government endorsement and funding of such emergency products.
There’s been much acerbic debate surrounding the Atlantic article by Emily Oster calling for a “pandemic amnesty” for those who pushed these demonic policies on the public. The piece was rightfully maligned by the public because the perpetrators of COVID fascism never even admitted they were wrong. But it’s worse than that. They haven’t even stopped the implementation of this iteration of biomedical terrorism and are already working on the next steps. They are still pushing these same shots and other similar ones in production without any liability. They are still blocking research and treatment of COVID and vaccine injury. They are still engaging in gain-of-function research. And they still have not even signed on to legislation ending the president’s ability to indefinitely declare a public health emergency, regulate human bodily autonomy, and discriminate against people for personal health care choices.
Thus, it’s no longer even about the malfeasance of lockdowns and mandates, as horrific as they were. They are still promoting death shots and the proliferation of the technologies, surveillance, and biomedical security apparatus that allowed them to enter the bodies of the supermajority of adults in the world. Every single biomedical product pushed on the American people – from the shots and Paxlovid to remdesivir – were extremely dangerous and destructive. Republicans cannot simply walk away from this without legislation imposing basic liability exposure on the drug makers, investigating those behind the maleficent approval, and preventing this from happening in the future.
We thought the era of human experimentation was buried with the Nuremberg trials, but clearly there is a need to reaffirm the Nuremberg Code with Nuremberg 2.0 to ensure that the worst pharma actors are not the ones with the greatest exemptions from human rights violations.


 Is there some sort of unholy union between the FDA, Pfizer, and the Biden White House?
Is there some sort of unholy union between the FDA, Pfizer, and the Biden White House?

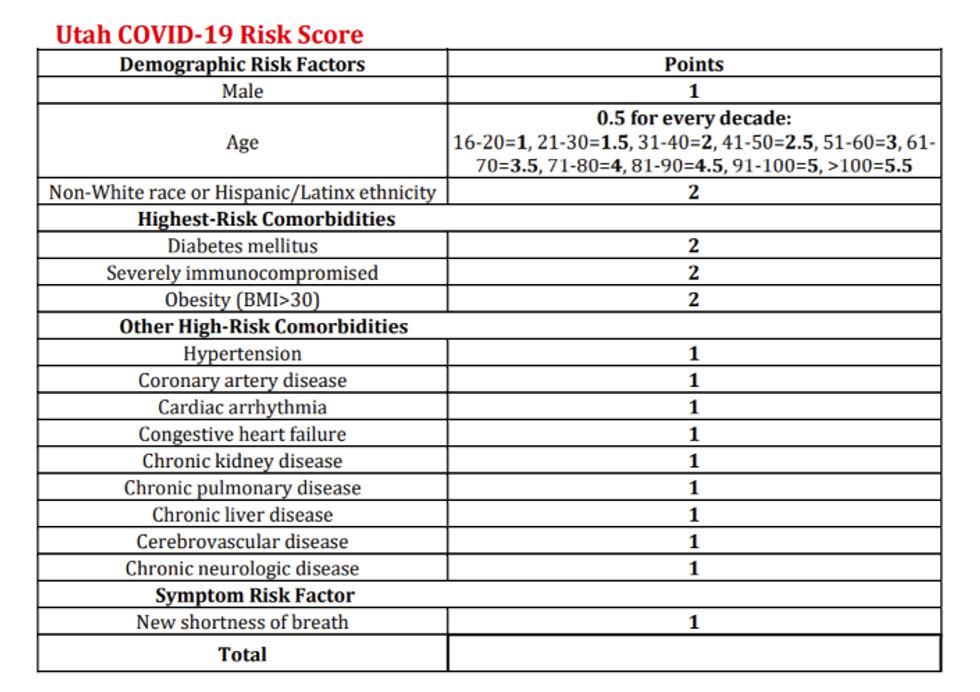 Utah Department of Health
Utah Department of Health